5.6 Water: A Polar Molecule
- Due Apr 24, 2020 at 11:59pm
- Points 4
- Questions 4
- Time Limit None
- Allowed Attempts Unlimited
Instructions
Polar & Non-Polar Molecules: Crash Course Chemistry #23
Polar Vs Non-Polar molecules
Let's take a look at 1 water molecule.




Remember when we talked about electronegativity we said that different elements have different electronegativities. Electronegativity is created because each elements have different affinities (loves) for electrons. In the case of water (H2O) notice that the electrons lie closer to oxygen. This creates partial negative and partial positive charges, creating what we call as polar molecules.

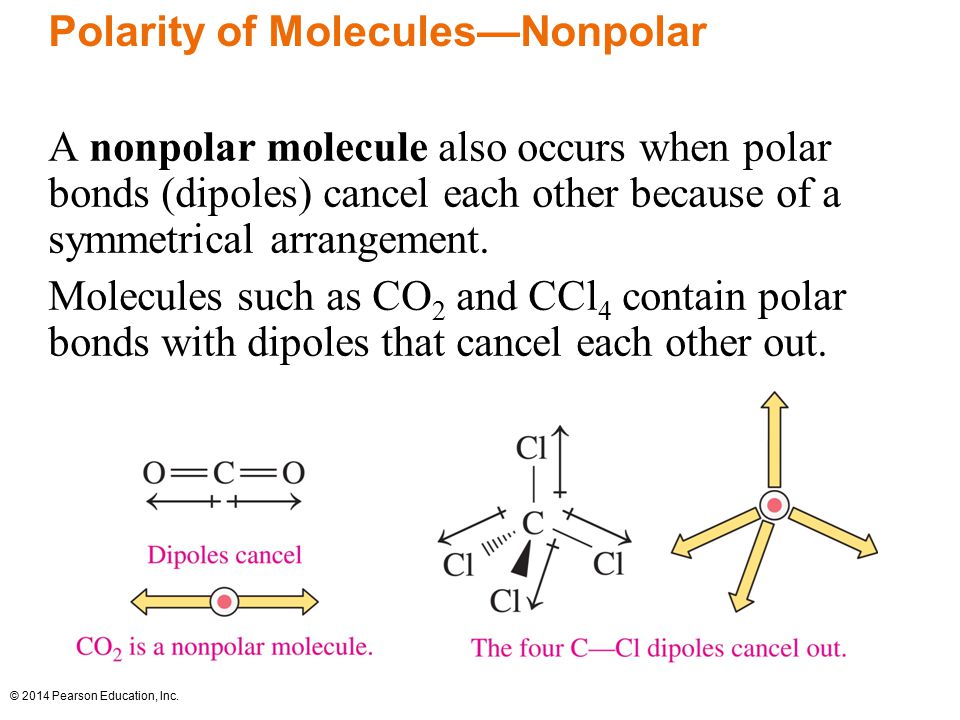
Differences in electronegativities creates dipoles (two poles). In the case of BF3, the three dipoles created cancel each other out. This creates a nonpolar molecule. Think of it like tug-a-war. If we have three things pulling at opposite directions there will be no movement.