5.6 Water: Hydrogen Bonding
- Due Apr 24, 2020 at 11:59pm
- Points 4
- Questions 4
- Time Limit None
- Allowed Attempts Unlimited
Instructions
Hydrogen Bonding and Common Mistakes
We call bonds between 2 different molecules intermolecular bonds. The prefix inter- means in between two different things. Think of the word “inter”national. That means between 2 different countries. This type of intermolecular bond is called a hydrogen bond. A hydrogen bond occurs between hydrogen and oxygen, nitrogen, or fluorine. The hydrogen bond you should be most familiar with is in water. Each molecule of water (H2O) interacts with 4 different water molecules.

Each water molecule interacts with 4 different water molecules through a hydrogen bond.
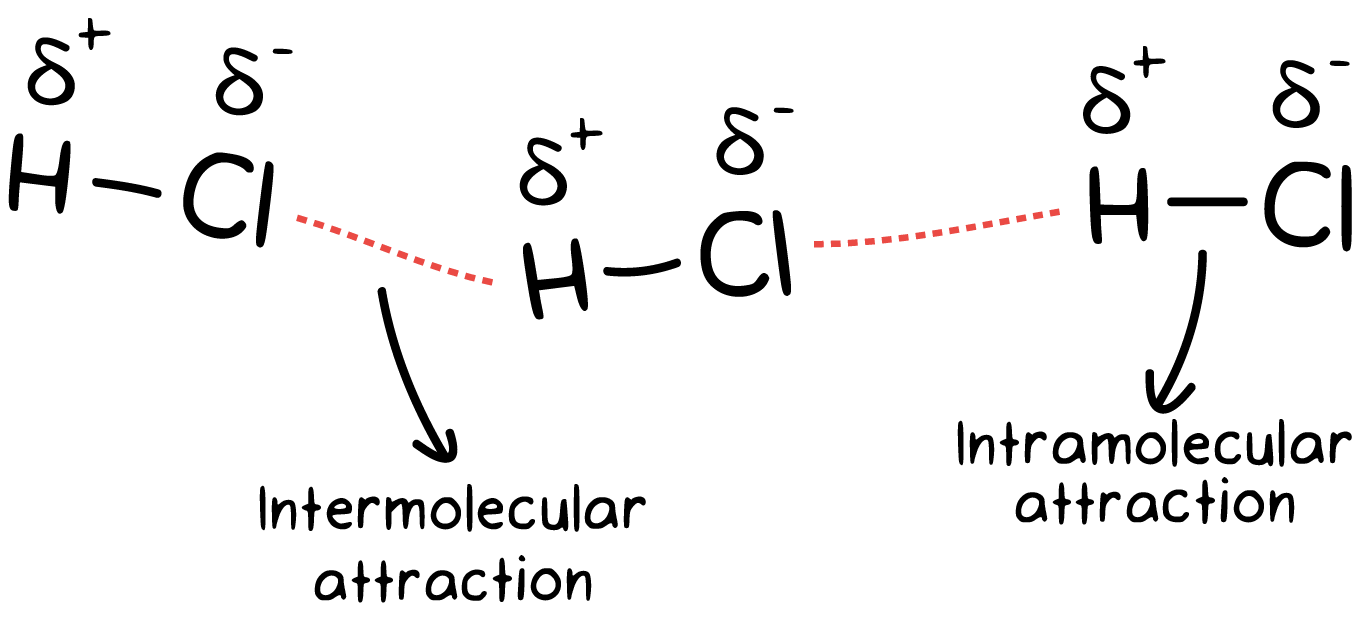
Notice: intermolecular forces are between 2 different molecules. Whereas, intramolecular forces are forces within a single molecule.
Only registered, enrolled users can take graded quizzes